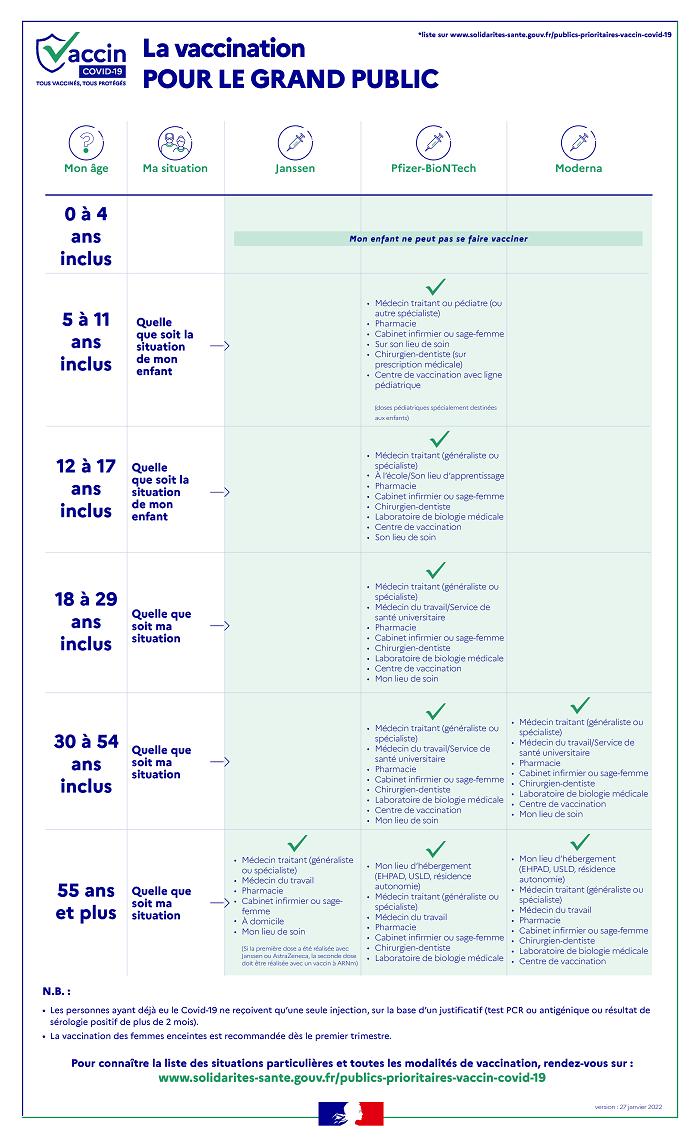
Covid-19: everything you need to know about pregnant vaccination
The vaccination campaign against Covid-19, which began in December 2020, is in full swing. Over the phases, first of all people at risk were vaccinated, then those aged over 18 without comorbidity, followed by children over 12 and more recently, 5-11 year olds. For pregnant women, the road to vaccination has been a long one. After a first vaccination authorization from the second trimester of pregnancy given on April 3, 2021, an extension from the start of pregnancy on July 20, the green light from gynecology and obstetrics professionals for a third dose on November 17 2021, it is finally since November 27, 2021 that they can display a complete vaccination schedule, as soon as they respect the minimum time between two injections.
In which trimester of pregnancy should you be vaccinated?
This is a question recently answered by American researchers from Weill Cornwell Medicine, in an article published on the institute's website and in the specialized journal Obstetrics & Gynecology, on December 28, 2021. After a large-scale study, they demonstrated that the rate of maternal antibodies were detectable after delivery, whether the vaccine was carried out before or during pregnancy. However, they noted some differences depending on the time of injection.
Thus, they found that antibody levels at delivery tended to be higher when vaccination took place in the third trimester. However, they also found that antibody levels at delivery were still high, and probably still protective, when vaccination took place early in pregnancy or even a few weeks before. They clarified that a booster late in pregnancy could increase antibody levels. For them, there is therefore no reason to delay vaccination after the birth of a child.
Conclusions which follow the analysis of 1,359 pregnant women, who declared having been vaccinated against Covid-19, during or up to six weeks before pregnancy, and who gave birth at NewYork-Presbyterian / Alexandra Cohen Hospital for Women and Newborns after 34 weeks of pregnancy or more. Of these, the 20 who reported receiving a booster dose during the third trimester had even higher antibody levels in maternal blood and cord blood.
“The message is that you can get vaccinated at any time during pregnancy and it will likely benefit you and your baby when it's born. said one of the study authors, Dr. Yawei Jenny Yang, assistant professor of pathology and laboratory medicine at Weill Cornell Medicine and pathologist at NewYork-Presbyterian/Weill Cornell Medical Center. "These study results are consistent with what we see with other maternal vaccines such as influenza and Tdap*, which when given during pregnancy protect both mother and baby," said Dr. Dr. Laura Riley, chair of the department of obstetrics and gynecology at Weill Cornell Medicine, and chief obstetrician and gynecologist at NewYork-Presbyterian/Weill Cornell Medical Center.
The researchers are now planning further studies to examine the effects of the vaccine and boosters in different maternity-related conditions, and in the context of the spread of the Omicron variant of SARS-CoV-2.
How to Release Sinus Pressure: Your sinuses are hollow, air-filled spaces inside your skull. Sinus pressure is... https://t.co/n2z49teiMm
— Phil Beale Tue Apr 19 21:38:19 +0000 2016
*the equivalent of the vaccine against diphtheria, tetanus and whooping cough, called DTaP in France.
A third dose of vaccine whatever the term of the pregnancy
The third injection is now offered to all pregnant women or those who wish to become pregnant. A decision dated November 27, 2021, which follows the opinion of the National College of French Gynecologists and Obstetricians (CNGOF) and the Research Group on Infections during Pregnancy (GRIG), published on November 17. Furious that the third injection was not offered to future mothers, they felt that the decline in immunity over time, coupled with the particular vulnerability of pregnant women to COVID, and all the more specifically in the event of comorbidity (chronic illness, immunosuppressive treatment, diabetes, hypertension, obesity, advanced age, etc.), were to lead to a reminder campaign: “a third dose of anti-SARS-CoV2 vaccine must be offered to women who wish to become pregnant or pregnant, regardless of the term of the pregnancy, when the initial pattern dates back more than 6 months,” they said at the time.
In their publication, these two reference institutions recalled that "compared to an uninfected pregnant woman, there is a risk multiplied by 18 of admission to intensive care, by 2.8 of fetal loss, by 5 of admission of the newborn in intensive care and more if comorbid. They also specified that vaccinated pregnant women were three and a half times less often infected and that no teratogenic effect or effect on the reproduction of the vaccines had been observed to date.
No risk for pregnant women vaccinated with messenger RNA
Today, pregnant women who so wish can therefore be vaccinated against Covid-19, at the rate of three doses. They are well and truly recognized as a group at risk of serious forms by the High Council for Public Health (HCSP) and the High Authority for Health (HAS), especially if there is a comorbidity.
Healthcare institutions prefer messenger RNA devices, such as Pfizer/BioNTech and Moderna for pregnant women. On January 18, 2022, the European Medicines Agency (EMA) thus declared that the studies on the anti-Covid-A9 vaccines of these two laboratories presented no risk for mothers and their babies. This assertion follows the conclusions of a working group of the European regulator, which reviewed and cross-checked a dozen studies carried out on a total of 65,000 future mothers. The statement states that "The review did not identify any signs of an increased risk of complications during pregnancy, miscarriages, premature births or adverse effects in unborn babies after vaccination with the Covid vaccine. mRNA". It also recalls that “the benefits of mRNA Covid vaccines during pregnancy outweigh any possible risks to pregnant women and unborn babies.” As for other licensed vaccines, the EMA said it would review their data "as it becomes available".
What we already know, however, and this since the summer of 2021, is that the Vaxzevria vaccine (better known as AstraZeneca) being an adenovirus vaccine, in other words, using a non-pathogenic virus to induce an immune response, is not recommended. In addition, the doses ordered by France from the Anglo-Swedish group have, for the most part, been transferred to the Covax international solidarity program (for developing countries). This follows the risks of thrombosis demonstrated on multiple occasions, and the cessation of deliveries to pharmacies and health establishments.
Many gynecologists and obstetricians in favor of vaccination
The involvement of gynecologists and obstetricians in favor of vaccination (under certain conditions) for pregnant women and women wishing to have a child continues. Already, in January 2021, many of them rose up against the precautionary principle aimed at vaccinating only future mothers from the second trimester. Thus, the National College of French Gynecologists and Obstetricians was in favor of the vaccination of pregnant women as a priority. For him, the Covid vaccines being messenger RNA and not of the live attenuated type had “no reason to be contraindicated”. The same goes for the Society of Obstetricians and Gynecologists of Canada (SOGC), which then published the following statement: "Vaccination should be offered to pregnant or breastfeeding women at all times if they are eligible and have no contraindications. -indication. They explained that the risk of infection or morbidity linked to the virus was theoretically greater than the risk linked to vaccination. They further added that, although pregnant women are excluded from clinical trials, some have slipped through the cracks. “Despite this exclusion, 23 women (12 in the vaccine group and 11 in the control group) reported being pregnant during the trial. These people are monitored for pregnancy outcomes. To date, no adverse effects have been reported,” the SOGC said at the time.
Read also: